Researchers at the University of Toronto have challenged the prevailing theory about cellular reprogramming by identifying neural crest stem cells in the skin and other areas as the primary source of reprogrammed neurons. Unlike the idea that any mature cell can be converted into an unrelated cell type through transcription factors, their findings suggest that rare, multipotential neural crest stem cells are uniquely capable of this transformation, which may explain the inefficiency often seen in reprogramming efforts.
The study, Neural crest precursors from the skin are the primary source of directly reprogrammed neurons, emphasizes that cell identity is more stable than previously thought, indicating that successful reprogramming is more likely to occur within cells derived from the same embryonic layer. The researchers specifically explore how developmental lineage and the maturity of starting cell populations affect the reprogramming process, using murine fibroblasts as a model. They propose that most reprogrammed neurons come from a specific lineage of neural crest cells, and their experiments show that when rare proliferating NC precursors are selectively removed, the number of reprogrammed neurons significantly decreases.
These findings, published in Stem Cell Reports, suggest that the process represents direct differentiation within a neural lineage stem cell, rather than a cross-layer conversion from mesoderm to ectoderm. The study highlights the potential of neural crest stem cells for stem cell therapies, given their widespread presence and reprogramming capability.
The main findings from this:
•
Neural crest cells are the source of most directly reprogrammed neurons
•
The neural crest bias for neuron production is cell autonomous
•
Removing neural crest precursors largely reduces reprogramming efficiency
•
This challenges the interpretation of directly reprogramming across germ layers
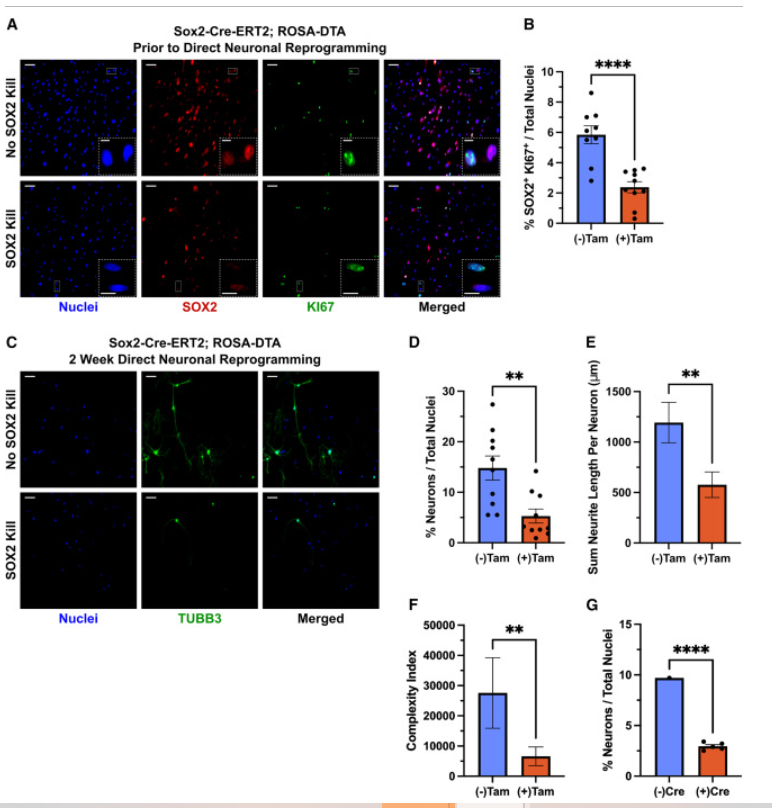 Figure 4. Epidermal Cells Rarely Reprogram to Induced Neurons (iNs)
Figure 4. Epidermal Cells Rarely Reprogram to Induced Neurons (iNs)
Figure 4. Epidermal Cells Rarely Reprogram to Induced Neurons (iNs)
(A) Shows images of skin cells from K15-CrePR1; tdTomato mice before reprogramming. Cells were treated with mifepristone to induce Cre for three passages.
(B) Quantifies the percentage of epidermal lineage cells before reprogramming. Data is from 9 wells (no mifepristone) and 16 wells (with mifepristone) across multiple embryos, with a significant difference (p < 0.0001).
(C) Quantifies the percentage of iNs before reprogramming in all mifepristone-treated cells, based on 13 wells from 5 embryos.
(D) Displays images of proliferating skin cells before reprogramming.
(E) Quantifies the percentage of proliferating epidermal and non-epidermal cells, showing no significant difference (p = 0.1737).
(F) Shows images of skin cells after 2 weeks of direct reprogramming.
(G) Quantifies the percentage of iNs in epidermal and non-epidermal lineages after reprogramming, with a significant difference (p < 0.0001).
(H) Measures the total length of neurites per iN, showing a significant difference (p = 0.0074).
(I) Measures the complexity index per iN, also showing a significant difference (p = 0.0012). Data includes 95 non-epidermal and 65 epidermal neurons.
Scale bars are 100 μm for main images and 25 μm for inserts. Error bars represent mean ± SEM.
MY THOUGHTS
I thought that these findings were interesting and possibly very beneficial for the future. Concerning stem cell therapies, this could be a good benefit as neural crest stem cells have more of a widespread presence and reprogramming capability.
Sites Used:
University of Toronto. "Researchers challenge longstanding theories in cellular reprogramming." ScienceDaily. ScienceDaily, 1 November 2024. <www.sciencedaily.com/releases/2024/11/241101123641.htm
https://www.sciencedirect.com/science/article/pii/S2213671124002911?via%3Dihub
No comments:
Post a Comment